Optical Flow is a measure of sub-pixel dislocations
of objects in a time lapse of images. Optical Flow is
velocity, in a vector or absolute value form, telling
for the pixels of an image that how fast and into what
direction do the underlying objects move (details).
Optical Flow is especially suitable for measuring motion in a
Multi-Dimensionalassays, because motion vectors are calculated from
pairs of images recorded shortly one after each other. Optical Flow allows
visiting multiple stage positions (x,y-coordinates) between time
points without an effect of inaccuracies in the stage positioning on
the motion. Therefore Optical Flow is discussed in the context of
Multi-Dimensionalimage acquisition (e.g. Metamorph Multi-DimensionalAcquisition, Elements ND acquisition, Zeiss LSM Multi Time
Series). Image Analyst MKII is an offline analysis tool, therefore
relies on recording done by image acquisition softwares.
Requirements for Optical Flow calculation:
- To calculate Optical Flow pairs of images (short
time lapses) are recorded with the same illumination
and exposure settings at the very same x,y,z position, with a
given time interval.
- The time points of the acquisition of both images have to be
accurately recorded; it is rather important to exactly know the
actual time interval between the images than setting accurately
a given interval (hardware delays can result altered acquisition
intervals).
- These pairs of images are recorded cyclically, in each time
point of the assay, for each stage position. This results a time
lapse of short time lapses (see Figure below).
- Images recorded typically at 512x512 pixels at ~0.2-0.3
mm/pixel resolution. There is no
limitation on image size for Optical Flow calculation, but
larger images will compute significantly slower.
- The noise characteristics of the sensor (CCD camera, PMT)
has to be measured in a set of evenly illuminated images at
different intensities. This must happen at identical camera or
PMT settings to the actual experiment.
Setting microscopy parameters
- Wavelength (selection of the fluorophore):
Optical Flow analysis is done typically well above the Rayleigh/Nyquist
limit, therefore there is no preference between fluorophores of
different emission wavelength. In additions
velocities/dislocation of edges is not affected by the
resolution limit.
- Resolution: For measurement of
mitochondrial motion we found that 0.2-0.3 mm/pixel
resolution was optimal1. Higher magnifications may be
affected by subtle changes like peristaltic-like motion of inner
mitochondrial membrane / cristae, which may not be visually
obvious, but generate Optical Flow.
- Acquisition interval: The dynamic range of
the Optical Flow is relatively narrow. It measures ~0.1-1.2
pixel/frame velocities (the bottom of the range is determined by
the actual signal to noise ratio). Therefore the acquisition
interval is determined by the expected velocities the
resolution. E.g. if 0.5 mm/s
velocities are expected and the resolution is set to 0.2
mm/pixel then the frame interval has
to be between 0.1*0.2/0.5=40ms and 1.2*0.2/0.5=480ms. If using
short time lapses more than two frames (which is not advised)
this time period spans the acquisition from the first to the
last frame of the short time lapse.
- Focus: Optical Flow can correctly detect
velocities of slightly off focus objects. So if some defocusing
happens gradually during a long time lapse that is a little
concern. However Optical Flow is highly sensitive for focal
changes between frames of the short time lapse. Therefore if
active, looped back focusing mechanism is used, it may have to
be turned off during the acquisition. Along the same line, it is
better to use larger pinhole for Optical Flow
acquisitions.
- Stage motors and vibration: Vibration causes
uniform error in Optical Flow over the whole image. However in
our practice, having microscopes in simple air tables, we did
not encounter artifacts accounted for vibration. Some motorized
stages with encoders and active loopback may flutter around the
set position. Servos should be turned off during Optical Flow
acquisition if this is a problem.
Image acquisition
Image acquisition softwares typically do not provide native
support for recording a time lapse of short time lapses, with the
exception of the Zeiss LSM Multi Time Series module. However, using
simple scripting and other 'tricks' short time lapse acquisition
can be achieved. This requires individual solutions for
each image acquisition environment. Multi-Dimensionalimage
acquisition for Optical Flow assays are designed as follows:
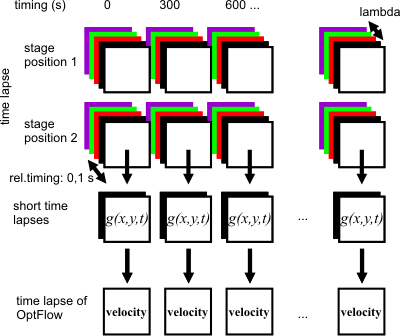 |
| Multi-Dimensionalacquisition: each lambda or spectral loop over
a stage position is finished by the acquisition of the
short time lapse (black and white squares). The
reconstituted Optical Flow time lapse for stage position
#2 is shown in the bottom. See details
here. |
Protocols are provided for image acquisition using the systems
below, followed by analysis in Image Analyst MKII:
- Molecular Devices Metamorph 6.3
- Nikon Elements 3.1
- Zeiss LSM Multi Time Series
Analyzing
simple time lapse recordings
- An image series is recorded in an arbitrary image
acquisition environment. The timing of each frame has to be
accurately saved. There is no stage movement between
frames, so all frames perfectly register (if using
block mode,
fames have to perfectly register within the blocks but not
between blocks)
- An image series loaded as an Image Window can be processed
as optical flow.
- If the recording was not saved as *.stk, *.lsm or *.nd2
file, the import of the timing of the experiment can be
problematic. If the acquisition interval was even, the time axis
can be entered using the Editing/
 New
Time Scale function.
New
Time Scale function.
- Background must not be subtracted. The
original background level is required masking of Optical Flow
images.
- Select the
 Optical
Flow function in the main menu Special are listed.
The following parameters may have to be set in the
parameter bar:
Optical
Flow function in the main menu Special are listed.
The following parameters may have to be set in the
parameter bar:
- Select dt kernel: [1,-1] or set the
length of blocks if the recording was in
block mode.
- If the block size is greater than 2, set [Savitzky-Golay
first derivative] here and enter the size of the block
at the SG kernel for dt width, and enter
No at #2 below.
- Average OF for dt width: Yes
(dt kernel of width of two always used with averaging to
avoid biasing between leading and trailing edges. Set No if
using wider kernel)
- Block mode: if the experiment was
recorded with an even frame rate around 1s/frame or less set
No. If the experiment was recorded as
frames (equal number of the width of the dt kernel at short
interval, then pause for an arbitrary time, and then this is
cyclically repeating, set Yes.
- Pixel size: (the mm/pixel
calibration can be given here to obtain velocities in
mm/s rather than in pixel/s. 1
results output in pixels/s. Use the
context menu
Show Image Info of an Image Window to determine
scaling)
- Output as... (enable the desired kind
of outputs; as default only absolute velocities are
calculated)
- Output as Absolute value of Projected Vectors:
If Yes, velocity vectors are projected to a
point ROI. This can be used to assess anterograde transport
(away from the point ROI) by positive velocities and
retrograde transport (towards the ROI) by negative values.
When using this feature first (before #3) load the image
series by setting the Processing panel to None
in the Open tab. Draw ROI on the opened image. Then
follow the above protocol form #3. Set the ROI No. in the
Projection ROI parameter. The ROIs are automatically copied
from the last open image during Optical Flow open.
- Other parameters: noise parameters were filled in above.
Fine tuning of other parameters see here.
- In the context menu of the Image Window click
Process This with Optical Flow.
- The default LUT of the Optical Flow image is pseudocolor,
and can be set in the
Preferences dialog.
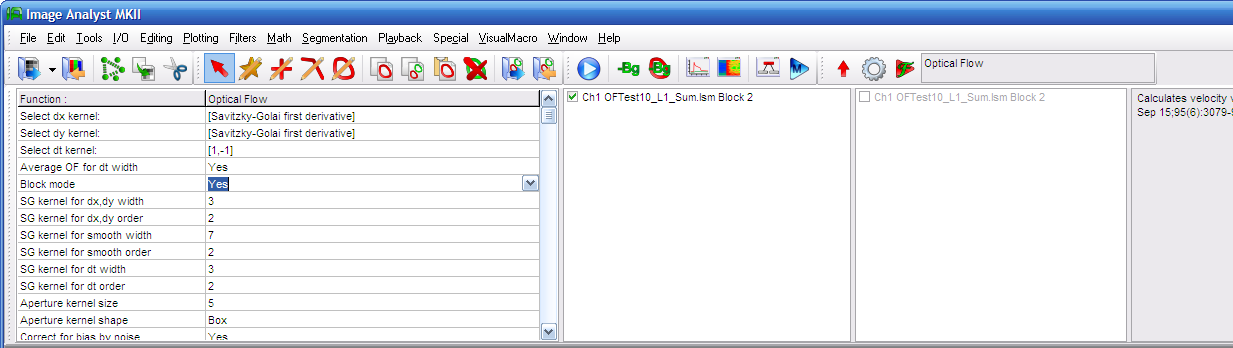 |
Select the
 Optical
Flow function in the Special menu. Optical
Flow function in the Special menu.
if the experiment was recorded with an even frame rate
around 1s/frame or less set No for the Block Mode. If the experiment was recorded as
frames (equal number of the width of the dt kernel at short
interval, then pause for an arbitrary time, and then this is
cyclically repeating, set Yes for the
Block Mode. |
Tuning analysis parameters
To perform Optical Flow analysis only the noise
parameters, Pixel size and
optionally the Block mode have to be set,
as given in the detailed protocols (links above), and the rest of
the parameters are used at their Default Values. Parameters
other than these should be altered only with the understanding the
compete Optical Flow algorithm.
More about tuning parameters.
See also Working
with Optical Flow Images
Protocol by Akos A. Gerencser 11/11/2009 V1.0
References
1.
Gerencser
A. A. and Nicholls D. G. (2008) Measurement of Instantaneous
Velocity Vectors of Organelle Transport: Mitochondrial Transport and
Bioenergetics in Hippocampal Neurons. Biophys J. 2008 Sep
15;95(6):3079-99.


![]()