Recording Optical Flow with the Nikon Elements software
Acquisition in Nikon Elements
Recording noise characteristics
The aim here is to record a set of evenly illuminated fields at
different intensities from zero to close to saturation. This have to
be performed at completely identical camera settings to the Optical
Flow recording.
- To record noise characteristics use completely identical
settings of the camera to the settings used in experiments.
- Lens and filters do not have to be identical.
- Mount a fluorescent plastic slide on the microscope. Select
a proper optical configuration for the fluorescence spectrum.
- Focus it to have as even illumination in the image as
possible.
Alternatively the objective lens can be removed and the slide is
placed on the nosepiece, in the back focal plane of the lens.
- Use the Acquire/Capture Time Lapse/Capture Manually feature of Nikon
Elements
- Start with zero illumination; simply acquire an image while
the light path is diverted from the camera, e.g. 100% to
eyepiece.
- Set light path to the camera and gradually increase
illumination, frame-by-frame. For this:
- Use the intensity control of the illumination unit, e.g.
Sutter Instruments Smart shutter
- or use the aperture diaphragm in the fluorescence
illumination pathway
- or pull out and then gradually push back the fiber
optics light guide
- It is sufficient to use illumination levels up to it results
in similar pixel intensities to the observed intensities during
experiments. Do not saturate.
- Save acquired set (time lapse) of images as nd2 file.
Recording Optical Flow in ND acquisition
The best way for Optical Flow recording in Nikon Elements is to
use a repeated channel in the Lambda tab of the ND Acquisition
dialog. The following two requirements have to be fulfilled:
- Accurate timing of Optical Flow frames is required, but
Elements does not record time points of the recording of each
image within a Lambda loop. However, Elements records
the time points of macro command executions, as experiment
events. This is set in the Advanced part of the
Lambda tab of the ND Acquisition.
- Sufficient delay has to be applied between acquiring Optical
Flow frames. This is achieved by a macro command in the Advanced part of
the Lambda tab of the ND Acquisition.
Settings in the ND Acquisition dialog of Nikon Elements
- Acquisition parameters should result (optimally) 512x512
pixels images at ~0.2-0.3 mm/pixel
resolution. For example:
- 60x lens, 1.0 Zoom, no binning of a 16mm
pixel camera: 16/60=0.267 mm/pixel
- 40x lens, 1.0 Zoom, 2x2 binning of a 6.4mm
pixel camera: 2*6.4/40=0.32 mm/pixel
- Select the Lambda tab of the ND Acquisition
dialog
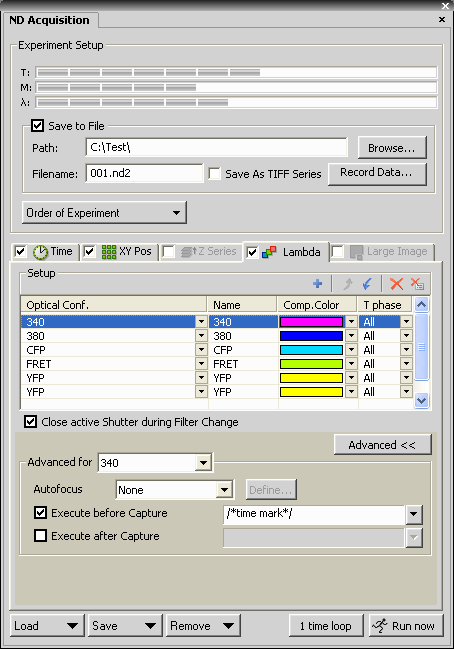
- Set up channels (optical configurations) to be acquired. The
channel used for Optical Flow measurement is added twice as the
two last channels.
The channel used for Optical Flow is simply the optical
configuration of the fluorophore that visualizes the moving
organelle, e.g. mito-GFP.
- Because the Optical Flow calculation depends on the noise
parameters of the camera, the gain, multiplier, AD conversion
clock (MHz) settings (if applicable for the camera in use)
should not be varied between experiments, unless the noise
characteristics is measured for each setting. The exposure time
may be varied. The safest to set intensities by varying
illumination intensities.
- Open the Advanced part of the dialog
- For each channel (optical configuration used), check the
Execute before capture checkbox, and enter a commented
expression e.g. /*time stamp*/
Commenting is done by slash-star star-slash bracketing. The
comment text has to be identical for all channels, this will be
detected by the Image Analyst. If using other macro commands,
these can precede or follow the comment, e.g. when using macro
commands to offset focus compensating for chromatic aberration.
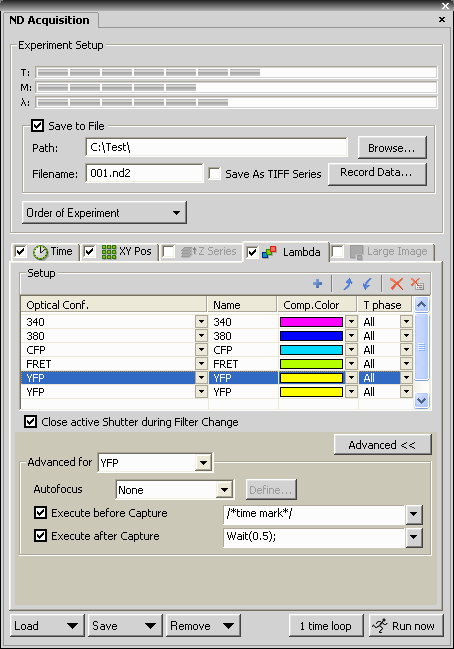
- For the first channel used for Optical Flow check the
Execute after capture checkbox, and type: Wait(0.5);
This sets up the interval between the two Optical Flow images.
The interval is given in seconds, and there is also a
significant hardware delay that adds to this delay. The value of
0.5 is an example here, it has to be set according the
application (see Gerencser & Nicholls 2008). To determine
real frame interval, use Image Analyst MKII, In
the Multi-Dimensional Open dialog, set:
- Open/Processing/Single Time Point
- ND2 Tweak/Use events for experiment timing...
and Load multiple channels as Z or OF
- Load a time point and look for the timing in the status
bar of the Image Window.
- The Time and XY Pos tabs can be arbitrarily set, while the
Z-Series and Large Image are disabled.
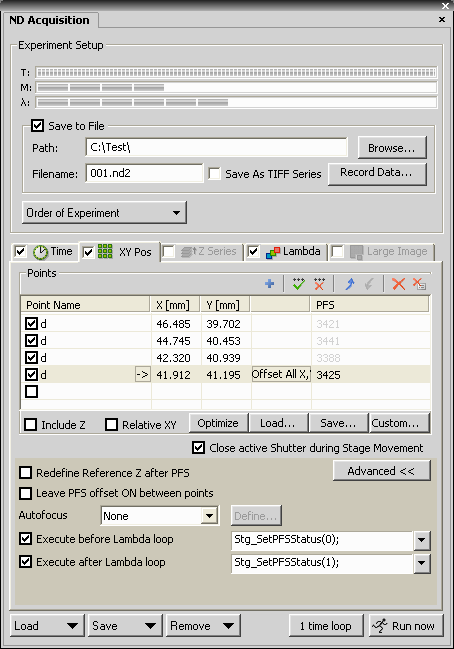
- It is advised to use the perfect focus system (PFS), or
other means of software auto focusing, if PFS is not available.
- If using PFS, it may be turned off for the duration of the
acquisition of the Optical Flow frames, to ensure that no focal
change happens due to fluttering of the active feedback
mechanism of the PFS. To turn it off change the
Execute before capture command of the first Optical Flow
channel to /*time stamp*/Stg_SetPFSStatus(0);
 |
 |
 |
|
In this example cells are stained by Fura-2-AM and
expressing mitochondrially targeted cameleon.
- Add the same comment e.g. /*time stamp*/ to each
channel Execute Before Capture.
|
The last channel (YFP) is repeated once, so the two
YFP channels will be used for Optical Flow calculation.
At the first Optical Flow channel set the Execute
After Capture to Wait(0.5); Use the proper wait time.
Expect a significant hardware delay in addition to the
exposure time.
|
If using the perfect focus system: In the XY Pos tab
set:
- Execute before Lambda Loop: Stg_SetPFSStatus(0);
to turn PFS off
- Execute after Lambda Loop: Stg_SetPFSStatus(1);
to turn PFS on
|
The protocol is based on Nikon Elements AR 3.0 and 3.1.
Analysis in Image Analyst MKII
Analyzing noise characteristics
- Open the noise characteristics file recorded above
- Set LUT scaling to frame-by-frame in the Set scaling
menu point of
context menu of the Image Window (check
Scale each frame independently)
- Look for a small part of the image where the illumination is
the most even. Draw a small ROI here (~20x20 pixels)
- Select the
 Sensor
Noise Characteristics in the Special main menu.
Sensor
Noise Characteristics in the Special main menu.
- In the Parameter Bar, set the 'Set values in
Optical Flow functions' parameter to Yes.
- In the context menu of the Image Window click
 Process This with Noise Characteristics; A Plot and a Text
window appear.
Process This with Noise Characteristics; A Plot and a Text
window appear.
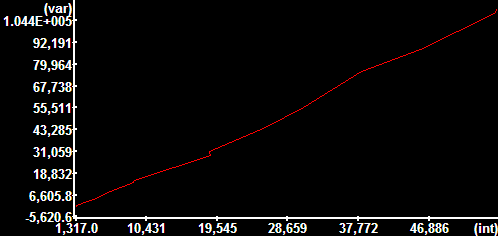
- The content of the Plot window is the intensity-variance
relationship of the pixels within the ROI. This has to be a
straight line. If it is not linear:
- Frames have to be in the order of increasing intensity
- Delete any saturated frames.
- Nonlinearity may be caused by uneven illumination. Move
the ROI around to find a linear spot.
- Try to draw a smaller ROI.
- The function automatically sets the following parameters of
the Optical Flow function:
- Detector offset (mean of the zero illumination image
intensity)
- Detector variance vs. intensity Slope (slope of the Plot
Window)
- Detector Read out Variance (variance at the zero
illumination)
- The above values will be stored when exiting Image Analyst,
or click Edit/Save Preferences in the main menu.
 |
 |
|
Noise curve of a Cascade 512B CCD camera
at binning: 1x1; Exposure: 100 ms; Multiplier: 2100;
Readout Speed: 5 MHz; Conversion Gain: 1/3 x;
Temperature: -30.1°C
The image on the left was scaled between its 1 and 99
percentiles, therefore shows inhomogeneities amplified.
Of note the readout noise show on the right is higher
than typical for this camera type, because of improper
Multiplier and Gain settings... |
Offset: 1,317.0
Variance vs. intensity Slope: 1.9810
Readout noise (variance σ2): 201.51
---------------------------------------------------
Electrons per gray unit: 0.5048
Readout noise (e-;RMS): 10.086 |
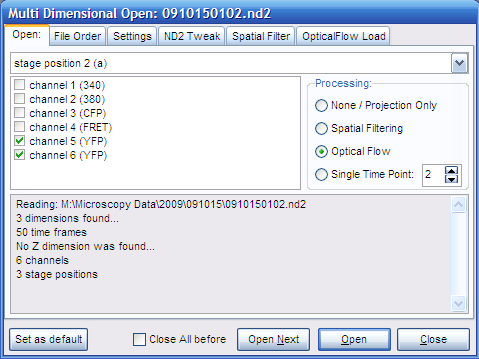
Analyzing Optical Flow
- Open the ND Acquisition file (*.nd2). If the experiment
consists multiple *.nd2 files they can be merged in time by
multiple selection. The Multi-Dimensional Open dialog appears.
- In Open tab: select only the two channels that will
be used for Optical Flow calculation. Select Optical Flow in the
Processing panel.
- In the Settings tab make sure that the Load
specified frames of each stack... and the Separate
Blocks... are not checked. The Load specified frames of
the time lapse feature can be used. If more frames
are processed than the width of the dt (temporal
differentiation) kernel, multiple velocity images are
calculated, and the result will be obtained by using projection
as given in the Project Z field.
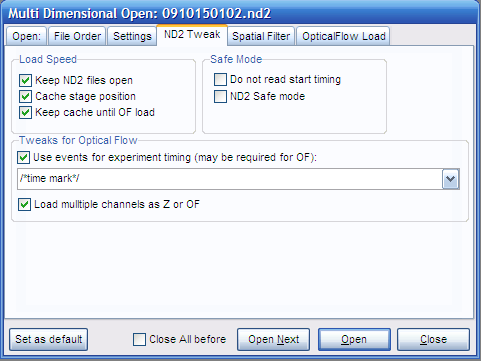
- In the ND2 tweak tab: check the Use events....
This feature is available only if macro command / comment events
were properly assigned before ND acquisition in the Elements.
Select below the actual time stamp, e.g. /*time stamp*/. Also
check Load multiple channels as Z or OF.
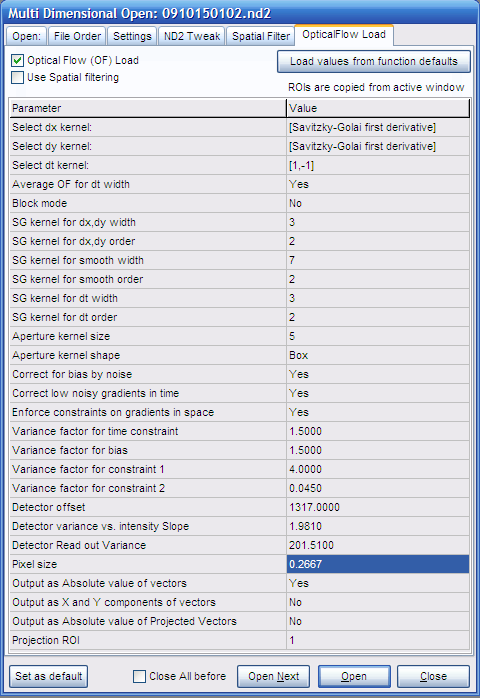
- In the OpticalFlow load tab the parameters of the
 Optical
Flow function are listed. The following parameters may have
to be set here:
Optical
Flow function are listed. The following parameters may have
to be set here:
- Select dt kernel: [1,-1] (to
match the length of short time lapses of two frames)
- If the block size is greater than 2, set [Savitzky-Golay
first derivative] here and enter the size of the block
at the SG kernel for dt width, and enter No
at #2 below.
- Average OF for dt width: Yes
(dt kernel of width of two always used with averaging to
avoid biasing between leading and trailing edges)
- Block mode: No (each
short time lapse is separately processed, so there is no
need for block mode)
- Pixel size: (the mm/pixel
calibration can be given here to obtain velocities in
mm/s rather than in pixel/s. 1
results output in pixels/s. Use the context menu
Show Image Info of an Image Window, or the
Tools/Setup DFT filter to determine
scaling)
- Output as... (enable the desired kind
of outputs; as default only absolute velocities are
calculated)
- Output as Absolute value of Projected Vectors:
If Yes, velocity vectors are projected to a
point ROI. This can be used to assess anterograde transport
(away from the point ROI) by positive velocities and
retrograde transport (towards the ROI) by negative values.
When using this feature first (before #2) load the image
series by setting the Processing panel to None
in the Open tab. Draw ROI on the opened image. Then
follow the above protocol form #2. Set the ROI No. in the Projection ROI
parameter. The ROIs are automatically copied from the last
open image during Optical Flow open.
- Other parameters: noise parameters were filled in above.
Fine tuning of other parameters see here.
- Above settings are valid as long as the dialog is open, or
can be stored by the Set as Default button.
- Click Open to perform loading and processing.
- The default LUT of the Optical Flow image is pseudocolor,
and can be set in the Preferences dialog.
The resultant Optical Flow image consists of pseudocolored pixels
where Optical Flow determination was feasible based on the noise
characteristics (there was enough image detail to distinguish
movement from noise), and black mask where not. The unit of the
Optical Flow image is pixel/s, or mm/s
if the Pixel size is set above.
 |
 |
 |
| |
Setting the /*time
stamp*/ and checking Load multiple channels as Z or OF
are crucial for Optical Flow processing. However uncheck
the Load multiple channels as Z or OF if
loading channels for analysis of fluorescence intensity. |
Analyzing Optical Flow from
simple time lapse recordings (see
figure about block mode)
- Open nd2 file in the File/Open image
series/measurement, and in the Multi-Dimensional Open dialog load the complete time lapse by
setting the Processing to None.
Importantly, this section is only valid for time lapses recorded
without stage movement.
- Background must not be subtracted. The
original background level is required masking of Optical Flow
images.
- Select the
 Optical
Flow function in the main menu Special are listed.
The following parameters may have to be set in the
parameter bar:
Optical
Flow function in the main menu Special are listed.
The following parameters may have to be set in the
parameter bar:
- Select dt kernel: [1,-1] or set the
width of blocks if the recording was in block mode.
- If the block size is greater than 2, set [Savitzky-Golay
first derivative] here and enter the size of the block
at the SG kernel for dt width, and enter
No at #2 below.
- Average OF for dt width: Yes
(dt kernel of width of two always used with averaging to
avoid biasing between leading and trailing edges. Set No if
using wider kernel)
- Block mode: if the experiment was
recorded with an even frame rate around 1s/frame or less set
No. If the experiment was recorded as
frames (equal number of the width of the dt kernel at short
interval, then pause for an arbitrary time, and then this is
cyclically repeating, set Yes.
- Pixel size: (the mm/pixel
calibration can be given here to obtain velocities in
mm/s rather than in pixel/s. 1
results output in pixels/s. Use the context menu
Show Image Info of an Image Window to determine
scaling)
- Output as... (enable the desired kind
of outputs; as default only absolute velocities are
calculated)
- Output as Absolute value of Projected Vectors:
If Yes, velocity vectors are projected to a
point ROI. This can be used to assess anterograde transport
(away from the point ROI) by positive velocities and
retrograde transport (towards the ROI) by negative values.
When using this feature first (before #3) load the image
series by setting the Processing panel to None
in the Open tab. Draw ROI on the opened image. Then
follow the above protocol form #3. Set the ROI No. in the
Projection ROI parameter. The ROIs are automatically copied
from the last open image during Optical Flow open.
- Other parameters: noise parameters were filled in above.
Fine tuning of other parameters see here.
- In the context menu of the Image Window click
Process This with Optical Flow.
- The default LUT of the Optical Flow image is pseudocolor,
and can be set in the
Preferences dialog.
 |
Select the
 Optical
Flow function in the Special menu. Optical
Flow function in the Special menu.
if the experiment was recorded with an even frame rate
around 1s/frame or less set No for the Block Mode. If the experiment was recorded as
frames (equal number of the width of the dt kernel at short
interval, then pause for an arbitrary time, and then this is
cyclically repeating, set Yes for the
Block Mode. |
Fine tuning optical flow (see
here)
Example
Example nd2 file (43MB, zip compressed)

Download and uncompress data on your hard drive.
- In the main menu select File/Open image
series/measurement, set the file type to "*.nd2" and open noise.nd2 in the “Noise
Characteristics” folder.
- Press Open in the appearing Multi-Dimensional Open dialog.
- Discard the last 3 frames because of saturation using the
 toolbar icon.
toolbar icon.
- Follow the points in the Analyzing noise
characteristics section above.
- Close images by File/Close all.
- In the main menu select File/Open image
series/measurement, set the file type to "*.nd2" and select
the file in the
“Mitochondrial Motion” folder.
- Set ND2 Tweak and and Optical Flow tabs as
shown above (the noise parameters should be automatically
entered by now)
- In the Settings tab uncheck everything.
- Switch back to the Open tab, select stage position
3 and only the two YFP channels and
Click Open. Inspect images.
- The Pixels size can be obtained in the
Tools/
 Setup
DFT Filter dialog.
Setup
DFT Filter dialog.
- Select Optical Flow in Processing and
press Open again.
- Draw a ROI around the neuron
 and press
and press
 .
.
- To analyze the Ca2+-imaging channels, first go
back to the ND2 Tweak tab and uncheck Load multiple channels as Z or OF
- In the Open tab select Processing None
and the first four channels. Click open.
- Click
 and subtract
background of the intensity images at 5 percentile from all
images.
and subtract
background of the intensity images at 5 percentile from all
images.
- Click
 to link intensity image to
the already open Optical Flow image.
to link intensity image to
the already open Optical Flow image.
- Use the Math/
 Ratio
to obtain Fura-FF 340/380 ratio or cameleon FRET/CFP ratio.
Proper FRET calculation from this data needs
spectral unmixing.
Ratio
to obtain Fura-FF 340/380 ratio or cameleon FRET/CFP ratio.
Proper FRET calculation from this data needs
spectral unmixing.
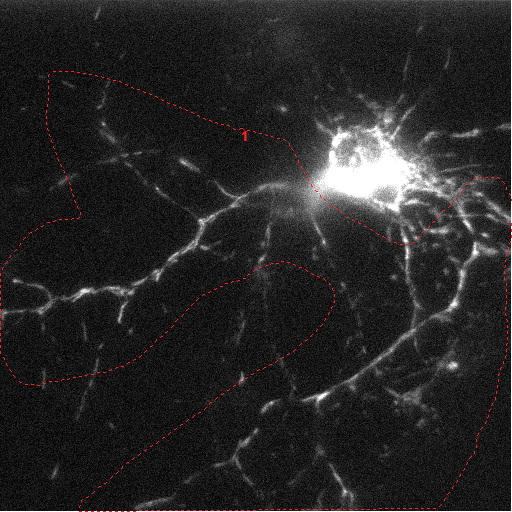 |
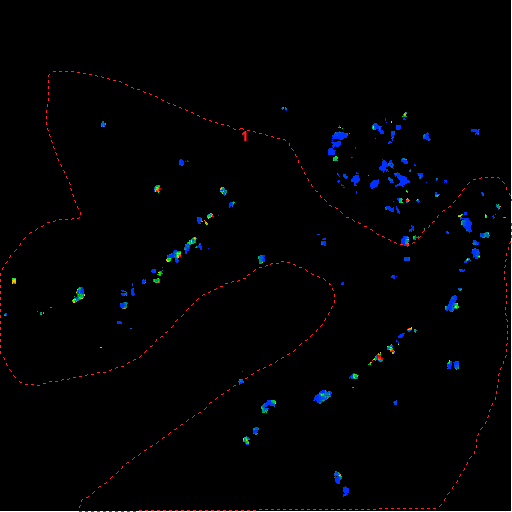 |
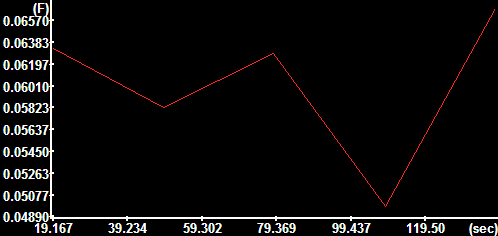 |
Frame 1 of
projection image of stage position 3
Hippocampal neuron expressing mito-D4cpv targeted
cameleon, acquired by a Nikon Ti2000 PFS |
Frame 1 of Absolute
Velocity Image |
Mean absolute
velocity over the encircled area in the images.
The y-axis is scaled in mm/sec. |
Protocol by Akos A. Gerencser 08/10/2010 V1.1
References
1.
Gerencser
A. A. and Nicholls D. G. (2008) Measurement of Instantaneous
Velocity Vectors of Organelle Transport: Mitochondrial Transport and
Bioenergetics in Hippocampal Neurons. Biophys J. 2008 Sep
15;95(6):3079-99.

![]()
![]()